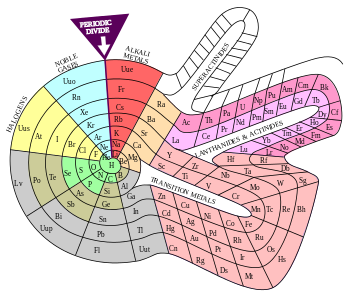
Alternative periodic tables
Alternative periodic tables are tabulations of chemical elements differing significantly in their organization from the traditional depiction of the Periodic System.[1][2] Several have been devised, often purely for didactic reasons, as not all correlations between the chemical elements are effectively captured by the standard periodic table.
Contents[hide] |
Aims[edit]
Alternative periodic tables are developed often to highlight or emphasize different chemical or physical properties of the elements which are not as apparent in traditional periodic tables. Some tables aim to emphasize both the nucleon and electronic structure of atoms. This can be done by changing the spatial relationship or representation each element has with respect to another element in the table. Other tables aim to emphasize the chemical element isolations by humans over time.
Major alternatives[edit]
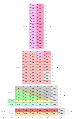
Charles Janet's Left Step Periodic Table (1928) is considered to be the most significant alternative to the traditional depiction of the periodic system. It organizes elements according to orbital filling and is widely used by physicists. Its modern version, known as ADOMAH Periodic Table, (2006) is helpful for writing electron configurations.[3]
|
Janet periodic table[4]
|
||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| f1 | f2 | f3 | f4 | f5 | f6 | f7 | f8 | f9 | f10 | f11 | f12 | f13 | f14 | d1 | d2 | d3 | d4 | d5 | d6 | d7 | d8 | d9 | d10 | p1 | p2 | p3 | p4 | p5 | p6 | s1 | s2 | |
| H | He | |||||||||||||||||||||||||||||||
| Li | Be | |||||||||||||||||||||||||||||||
| B | C | N | O | F | Ne | Na | Mg | |||||||||||||||||||||||||
| Al | Si | P | S | Cl | Ar | K | Ca | |||||||||||||||||||||||||
| Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | Rb | Sr | |||||||||||||||
| Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | Cs | Ba | |||||||||||||||
| La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | Fr | Ra | |
| Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | Uue | Ubn | |
|
|
||||||||||||||||||||||||||||||||
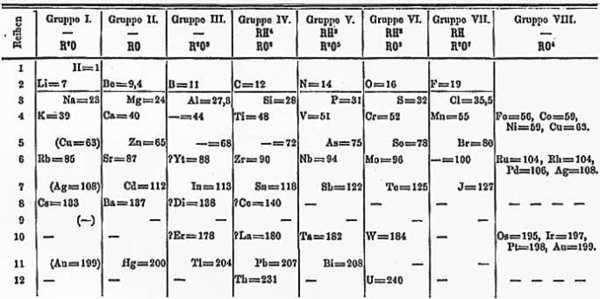
H.G. Deming used the long periodic table in his textbook General Chemistry, which appeared in the USA for the first time in 1923 (Wiley), and was the first to designate the first two and the last five Main Groups with the notation "A", and the intervening Transition Groups with the notation "B".
The numeration was chosen so that the characteristic oxides of the B groups would correspond to those of the A groups. The iron, cobalt, and nickel groups were designated neither A nor B. The Noble Gas Group was originally attached (by Deming) to the left side of the periodic table. The group was later switched to the right side and usually labeled as Group VlllA.
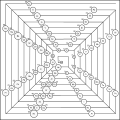
In Theodor Benfey's periodic table (1964), the elements form a two-dimensional spiral, starting from hydrogen, and folding their way around two islands, the transition metals, and lanthanides and actinides. A superactinide island is already slotted in.[5] The Chemical Galaxy (2004) is organized in a similar way.
In the extended periodic table, suggested by Glenn T. Seaborg in 1969, yet unknown elements are included up to atomic number 218. Helium is placed with the group 2 elements.
The oldest periodic table is the short form table of Dmitri Mendeleev, which shows secondary chemical kinships. For example, the alkali metals and the coinage metals (copper, silver, gold) are in the same column because both groups tend to have a valence of one. This format is still used by many, as shown by this contemporary Russian short form table which includes all elements and element names until roentgenium.
Timmothy Stowe's physicist's periodic table is three-dimensional with the three axes representing the principal quantum number, orbital quantum number, and orbital magnetic quantum number. Helium is again a group 2 element.
Paul Giguère's 3-D periodic table consists of 4 billboards with the elements written on the front and the back. The first billboard has the group 1 elements on the front and the group 2 elements at the back, with hydrogen and helium omitted altogether. At a 90° angle the second billboard contains the groups 13 to 18 front and back. Two more billboard each making 90° angles contain the other elements.[6][7]
In the research field of superatoms,
clusters of atoms have properties of single atoms of another element.
It is suggested to extend the periodic table with a second layer to be
occupied with these cluster compounds. The latest addition to this multi-story table is the aluminium cluster ion Al−
7, which behaves like a multivalent germanium atom.[8]
Ronald L. Rich has proposed a periodic table where elements appear more than once when appropriate.[9] He notes that hydrogen shares properties with group 1 elements based on valency, with group 17 elements because hydrogen is a non-metal but also with the carbon group based on similarities in chemical bonding to transition metals and a similar electronegativity. In this rendition of the periodic table carbon and silicon also appear in the same group as titanium and zirconium. A chemists' table ("Newlands Revisited") with an alternative positioning of hydrogen, helium and the lanthanides was published by EG Marks and JA Marks in 2010.[10]
Gallery[edit]
References[edit]
- ^ E.R. Scerri. The Periodic Table, Its Story and Its Significance. Oxford University Press, New York, 2006, ISBN 0195345673.
- ^ Henry Bent. New Ideas in Chemistry from Fresh Energy for the Periodic Law. AuthorHouse, 2006, ISBN 978-1-4259-4862-7.
- ^ Stewart, Philip J. (2009). "Charles Janet: Unrecognized genius of the periodic system". Foundations of Chemistry 12: 5. doi:10.1007/s10698-008-9062-5.
- ^ WebElements : The Janet Periodic Table.
- ^ Benfey's table appears in an article by Glenn Seaborg, "Plutonium: The Ornery Element", Chemistry, June 1964, 37 (6), 12–17, on p. 14.
- ^ Mazurs, E.G. (1974). Graphical Representations of the Periodic System During One Hundred Years. Alabama: University of Alabama Press. p. 111. ISBN 978-0-8173-3200-6.
- ^ The animated depiction of Giguère's periodic table that is widely available on the internet (including from here) is erroneous, as it does not include hydrogen and helium. Giguère included hydrogen, above lithium, and helium, above beryllium. See: Giguère P.A. (1966). "The "new look" for the periodic system". Chemistry in Canada vol. 18 (12): 36–39 (see p.37).
- ^ Amato, Ivan (November 21, 2006). "Beyond The Periodic Table Metal clusters mimic chemical properties of atoms". Chemical & Engineering News.
- ^ Rich, Ronald L. (2005). "Are Some Elements More Equal Than Others?". J. Chem. Educ. 82 (12): 1761. doi:10.1021/ed082p1761.
- ^ Marks, E. G.; Marks, J. A. (2010). "Newlands revisited: A display of the periodicity of the chemical elements for chemists". Foundations of Chemistry 12: 85. doi:10.1007/s10698-010-9083-8.
Further reading[edit]
- A 1974 review of the tables then known is considered a definitive work on the topic: Mazurs, E. G. Graphical Representations of the Periodic System During One Hundred Years. Alabama; University of Alabama Press, 1974, ISBN 0-8173-3200-6.
- Hjørland, Birger (2011). The periodic table and the philosophy of classification. Knowledge Organization, 38(1), 9–21.
External links[edit]
- QR-coded audio Periodic Table of the Elements: a mobile-learning tool
- Representing the Periodic Table in Different Ways a site curated by the Michigan State University Alumni Association Knowledge Network
- Robert Harrison's modern spiral periodic table
- Janet's Left Step Periodic Table
- Correction to Physicist periodic table offered by Jeries Rihani as Meitnerium occupies the position that Hassium should have.
- A Wired Article on Alternate Periodic Tables
- A Selection of Periodic Tables
- http://periodicspiral.com/ arranges the periodic table in a (hexagonal) spiral.
- Rotaperiod.com A new periodic table.
- Note on the T-shirt topology of the Z-spiral.
- New Periodic Table of the Elements this is in a square-triangular periodic arrangement.
- Periodic Table based on electron configurations
- Database of Periodic Tables
- Periodic Fractal of the Elements
- Bob Doyle Periodic Table of the Elements A regrouping by properties that suggests a maximum of 120 elements.
- Earth Scientist's Periodic Table
|
|||||||||||||||||||||||||||||||||||||||||||||||